Ultra-
Committed
We are committed to advancing innovative,
life-changing treatments
- Three of our medicines—CRYSVITA®, MEPSEVII®, and DOJOLVI®—are the only approved therapies for their respective diseases
- We engage those living with rare diseases and their caregivers to understand how these diseases affect their lives and to inform our efforts to develop treatments that will meaningfully benefit them
- We utilize innovative techniques—such as adaptive trial designs and seamless Phase 1/2/3 trials—to accelerate the drug development process
Innovation borne of urgency:
Matthew’s story

Innovation borne of urgency:
Matthew’s story
At 17 months old, Matthew was diagnosed with mucopolysaccharidosis type VII (MPS VII). His mother approached Dr. Emil Kakkis, our President and CEO, in 2013 when Matthew’s lungs were failing. Not expected to live long enough to participate in a clinical trial, Matthew was treated on a compassionate use basis with an investigational therapy developed by Ultragenyx. Matthew became the first person with MPS VII to be treated. Now 21 years old, Matthew is a loving person with an infectious smile who enjoys time spent with family and friends.
First-ever rare disease treatments at a speed faster than the industry average
Average number of years from entering the clinic to approval compared to ~7 to 7.5 years for our peers
Historical success rate in developing therapies from clinical study initiation to receiving commercial approval from regulatory authorities
We strive for global majority access
- We price our medicines responsibly and ensure that no one with a rare or ultrarare disease in the U.S. forgoes treatment for financial reasons (e.g., inability to afford copays or lack of insurance)
- We provide early pre-approval access to our therapies when medically appropriate

We only increase the prices of our medicines in a manner consistent with inflation adjustments
~50 countries where patients have access to our treatments

>600 patients have been approved for access to Ultragenyx treatments through various global expanded access and patient assistance programs since 2013
We support the rare disease community
We are committed to helping those living with rare diseases obtain early and accurate diagnoses whether or not they have a disease that we are studying. We share our science and expertise to advance treatment development, whether it’s through Ultragenyx or others in the rare disease community.
- We share de-identified natural history data, as well as endpoints, methods, and validations we have developed, with both physician groups and patient groups
- We host an annual bootcamp designed for patients and advocates who have started funding rare disease research and are looking to better coordinate and build structure around their efforts
- A prior boot camp attendee went on to found GeneTx Biotherapeutics, which we partnered with—and recently acquired—to develop their investigational drug to treat Angelman syndrome
participants representing ~100 organizations have attended the Rare Bootcamp™ since it’s 2017 inception
Access to our therapies

Access to our therapies
We believe we have an obligation to ensure global majority access to those living with rare and ultrarare diseases who can benefit from our medicines. We price our medicines responsibly, and in the U.S., we ensure that no one with a rare or ultrarare disease forgoes treatment for financial reasons.
Learn moreResources for patients and caregivers
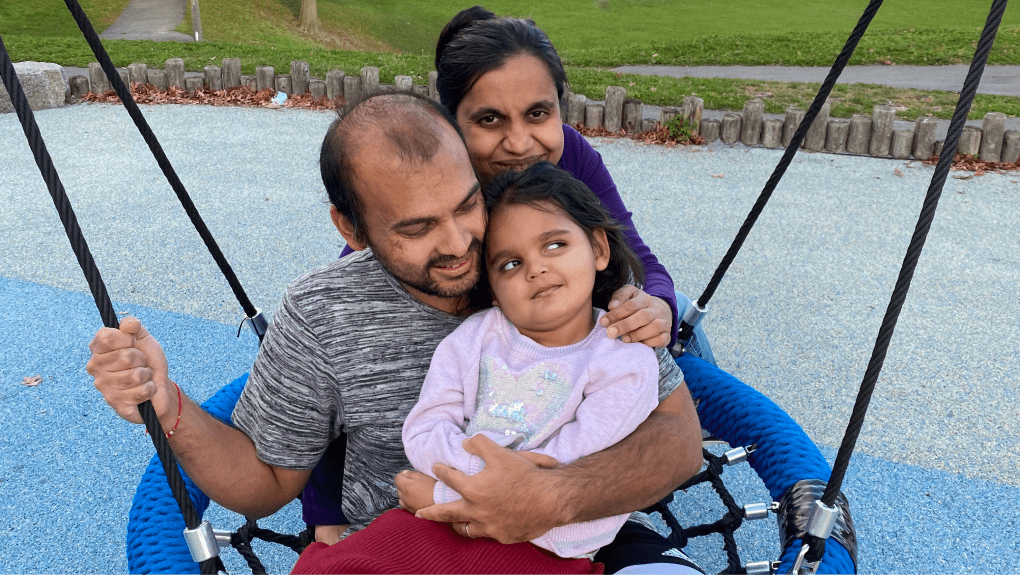
Resources for patients and caregivers
We strive to help all those with rare or ultrarare diseases access the treatments they need.
Learn more about early access

